Learn about Kringle Pharma
Quick Guide to Kringle and HGF
Who is Kringle Pharma?
- Corporate Philosophy
- We will contribute to the society and global health by research,
development and commercialization of innovative medicines for patients suffering from incurable diseases.
We focus our managerial resources on development of recombinant human HGF protein, in the belief that it will lead to therapeutic innovation, creating business opportunities and maximizing the value of the company.
To ensure the outcomes of its development are commercialized into pharmaceuticals that contribute meaningfully to society, our basic policy is to secure marketing approval on our own through in-house development primarily in Japan.
Kringle Pharma is a biopharmaceutical biotech focused on the development of HGF medicine
- Established in
- 2001
- Clinical-stage pipelines
- 4
(2 programs in Phase III)
- Patents
- 24
(including 3 patent applications)
as of September 2025
- R&D expenses
- 680 million yen
(fiscal year ended September 2025)
- Equity ratio
- 61.5% (as of the end of September 2025)
Kringle Pharma is a biopharmaceutical company founded as a biotech startup based on the research findings at Osaka University and Keio University. We are the only company in the world who have capability to mass-produce GMP-compliant, pharmaceutical-grade HGF (hepatocyte growth factor) for clinical use and have multiple clinical-stage pipelines. We aim to provide novel HGF therapies to the world for patients suffering from intractable diseases for which there is no truly effective treatment.
What is HGF?
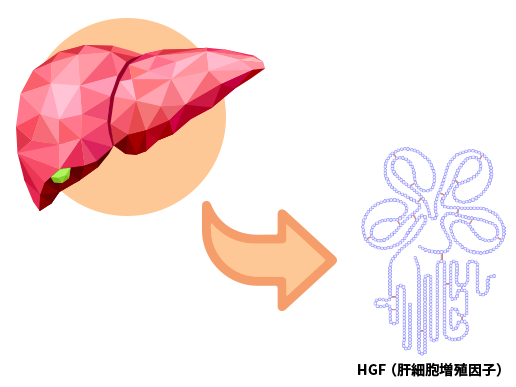
Originally discovered in Japan, HGF is a protein in human body that regenerates the liver
It has long been known that the liver has a strong regenerative capacity. For example, even if two-thirds of the human liver is removed, it eventually regenerates to its original size in about one month.
In 1984, HGF (hepatocyte growth factor) was identified by the late Professor Emeritus Toshikazu Nakamura of Osaka University as the principal factor contributing to the liver regeneration.
HGF protects, regenerates and repairs tissues and organs
HGF, discovered by Professor Nakamura, was later found to play a key role in the regeneration and repair of not only the liver but also various other organs and tissues and has a powerful effect on the nervous system.
On animal disease models, recombinant human HGF protein or HGF expression vectors have demonstrated the effectiveness in the prevention or treatment of a range of diseases for which no effective treatments were available, These included acute diseases (e.g. spinal cord injury, fulminant hepatitis, acute kidney injury, and myocardial infarction) as well as chronic diseases (e.g. cirrhosis, chronic kidney disease, pulmonary fibrosis, dilated cardiomyopathy, Huntington’s disease, and amyotrophic lateral sclerosis).
Diseases with potential clinical application of recombinant human HGF protein

-
- Kidney
- Acute kidney injury, chronic renal failure, renal transplantation, diabetic nephropathy
-
- Liver
- Acute hepatitis, fulminant hepatitis, liver cirrhosis, biliary atresia, fatty liver, liver transplantation
-
- Heart and blood vessels
- Vascular disease (arteriosclerosis obliterans, vascular restenosis prevention, etc.), myocardial infarction, dilated cardiomyopathy
-
- Nervous system
- ALS, acute phase spinal cord injury, cerebral infarction, Parkinson’s disease, Huntington’s disease, dementia
-
- Lungs and bronchi
- Chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, bronchial asthma
-

- Others
- Skin ulcer,vocal fold scar, inflammatory bowel disease, corneal injury
*Red text indicate target diseases for which we are currently developing drugs
Kringle Pharma named after HGF’s “Kringle” structure
An HGF protein has a complex molecular structure consisting of 692 linked amino acids. The molecule has four “Kringle” structures. A “Kringle” is a characteristic structure that resembles the kringle, a traditional Danish pastry.
Kringle Pharma is named after this “Kringle” structure, combined with “Pharma” to indicate it is a pharmaceutical company.

Development Pipeline
We have four pipelines that have progressed to clinical trials: acute phase spinal cord injury, vocal fold scar, ALS, and acute kidney injury. Phase III studies, the final stage of clinical trials, are underway in acute phase spinal cord injury and vocal fold scar. Claris Biotherapeutics, Inc. our partner in the US, is conducting a clinical trial in the US and Canada for ophthalmic diseases. In addition, we have several pipelines in the basic research stage. Currently, we are focusing our resources on drug development for acute phase spinal cord injury, which is at the most advanced stage of development, to obtain manufacturing and marketing approval. Meanwhile, for basic research, we are actively conducting joint research projects with universities and other institutions to expand indications and search for new seeds.
Now Kringle Pharma develops drugs for:

Acute Spinal Cord Injury(Acute SCI)

Vocal fold scar

ALS
Acute kidney injury
Diseases for joint research

Ophthalmic diseases

Other neurological diseases

Exploratory stage
Pipeline development status
| Target disease | Development stage | Basic research | Non-clinical study | Clinical study | Application/approval | Commercial | ||
|---|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | ||||||
| Acute Spinal Cord Injury (Acute SCI) |
Phase I/II study (placebo-controlled, double-blind, comparative study) completed, POC obtained, orphan drug designated, and phase III study (single-arm, open-label study) completed Additional study plan in progress |
Completed | CompletedAdditional study Planned |  |
||||
| Vocal fold scar | Phase I/II study (open-label dose-escalation study, investigator-initiated study) completed, Phase III study (placebo-controlled double-blind comparative study) ongoing. | Completed | Ongoing |  |
||||
| ALS | Phase II study (placebo-controlled double-blind comparative study, investigator-initiated study) completed, no statistically significant differences in primary or secondary endpoints, additional analysis ongoing. | Completed | Completed |  |
||||
| Acute kidney injury | Phase Ia, b Study (Open-Label Dose-Escalation Study) completed. Safety and pharmacokinetics confirmed. Partner search ongoing. | Completed | Searching for a partner | |||||
| Ophthalmic diseases | Development by Claris Biotherapeutics in the US (HGF supplied by Kringle Pharma) Phase I/II study in progress |
Completed | Ongoing | |||||
| Other neurological diseases | Joint research with Keio University | Ongoing | ||||||
| Exploratory stage | Joint researches with Kanazawa University, Kyoto University, etc | Ongoing | ||||||
As of January, 2026
Please click below for more information about our research and development.
HGF Potentials
Potential market size
The numbers of patients are limited as they are patients with acute phase spinal cord injury, vocal fold scar, and ALS, which are rare diseases. However, biopharmaceutical products like HGF are generally priced higher compared to small molecule products. HGF medicine is likely to be used widely once launched because there are few competitive products for those rare diseases, Accordingly, the market size of HGF worldwide is expected to reach tens of billions of yen for a single target indication.
| Target disease | Number of patients*1 | Competitor products | HGF market size*3 | ||
|---|---|---|---|---|---|
| Japan | World total*2 | ||||
| Neurological diseases | Acute Spinal Cord Injury (Acute SCI) | 5,000 | 60,000 | Stemirac, cell therapy, etc. * Combination with HGF considered |
|
| ALS | 9,800 | 85,000 | Riluzole, Edaravone, etc. * Combination with HGF considered |
|
|
| Fibrosis diseases | Vocal fold scar | 12,000 | 100,000 | Laryngoplasty, etc. (limited effect) * HGF will be the only drug therapy |
|
-
- *1 Sources:
- Our estimates based on Medscape Reference HP, Japan Intractable Diseases Information Center HP, The National Spinal Cord Injury Association HP, DATAMONITOR report, ASAKURA Internal Medicine 10th edition, published papers, etc., and “World Population Trends” by Statistics Bureau, Ministry of Internal Affairs and Communications
-
- *2
- Developed countries where advanced treatment is available
-
- *3
- Our forecast: calculated based on expected number of patients, diagnosis rate, treatment rate, drug utilization rate, and drug prices
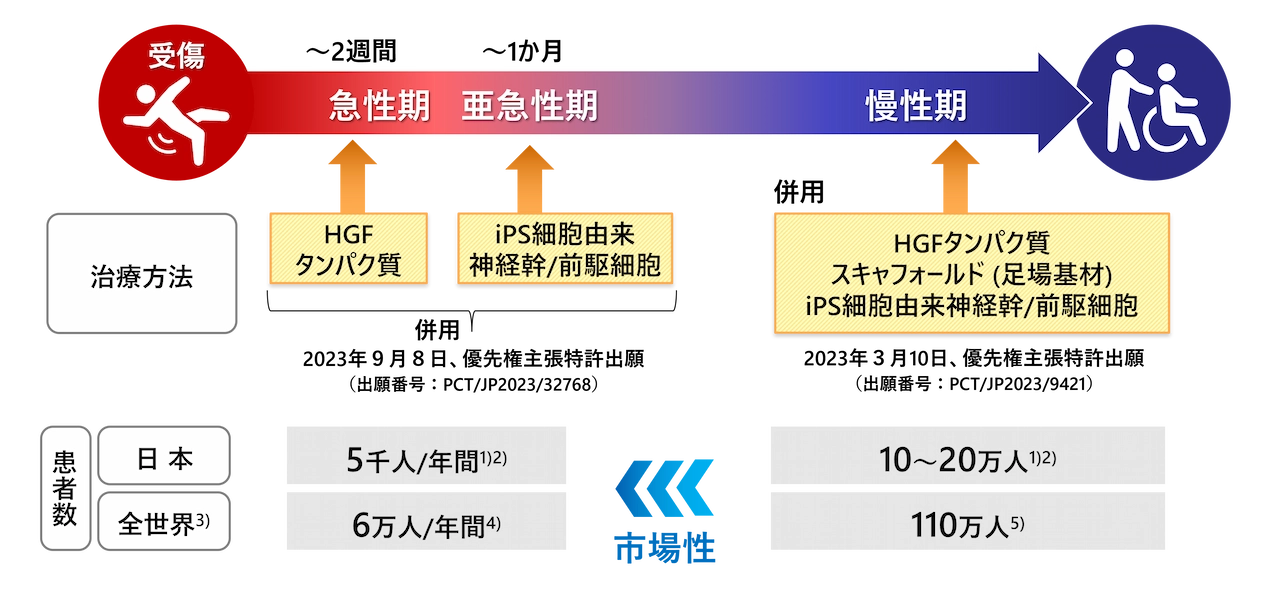
Expansion from acute injury phase to chronic injury phase of spinal cord injury
Through collaborative research with the Keio University School of Medicine, we aim to develop new therapies not only for the acute phase, but also for the subacute and the chronic phase of spinal cord injury. Expanding the indication to the chronic phase, the target number of the patients will significantly increase, and it will enlarge the market by at least 20 times.
-
- 1)
- Hikosuke Shingu, Prevention of Spinal Cord Injury, Journal of the Japan Medical Society of Paraplegia 13:48-49, 2000
-
- 2)
- Hiroaki Sakai, Survey for spinal cord injuries in Fukuoka. Sogo rehabilitation 36: 969–972, 2008
-
- 3)
- Developed countries where advanced treatment is available
-
- 4)
- Company estimates based on the number of patients in Japan, Spinal Cord Injury Facts and Figures at a Glance (2021), and Global Population Trends by the Statistics Bureau of the Ministry of Internal Affairs and Communications (MIC)
-
- 5)
- Company estimates based on Spinal Cord Injury Facts and Figures at a Glance (2021), The International Spinal Cord Injury Society Website, and Global Population Trends by the Statistics Bureau of the MIC
Expansion from vocal fold scar to other fibrosis diseases
If we are able to bring an HGF medicine to the world in vocal fold scar, we believe this will pave the way for a wider application of HGF to other chronic diseases caused by fibrosis. These fibrosis diseases have a larger number of patients and there are huge market needs and potentials.

-
- *1
- Calculated based on prevalence in Japan
-
- *2
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 545-602.
Future of Kringle Pharma
On top of the pipeline in neurological diseases (acute phase spinal cord injury and ALS), Kringle Pharma will promote development in fibrosis diseases (vocal fold scar) to better serve patients and maximize the long-term business value.

Our desire is, to provide innovative HGF medicines as soon as possible to bring smiles to patients and their families.