Research & Development
Research & Development
Drug candidate
Our primary asset for drug development is recombinant human HGF (“HGF protein”), and we are conducting clinical trials on HGF protein with several diseases.
HGF was initially discovered in Japan as a growth factor for hepatocytes. Growth factors are endogenous proteins that transmit signals to the nuclei (genetic material) of cells by binding to receptors on the cell surface, thereby turning on the switch that initiates cell proliferation. Because HGF is a naturally occurring substance that functions in the human body, the ability to artificially manufacture it in the form of recombinant human HGF protein will likely open the door to a new range of safe and effective pharmaceuticals.
Characteristics of HGF (Hepatocyte Growth Factor)

- A protein discovered in Japan that is endogenous to the human body
- Relatively large molecule of 692 amino acids
- Complex structure (called Kringle structure: origin of the company name), 19 intramolecular disulfide cross-links
- Multiple biological functions
- Protects, regenerates, and repairs tissues and organs
Mechanism of action of HGF (Hepatocyte Growth Factor)
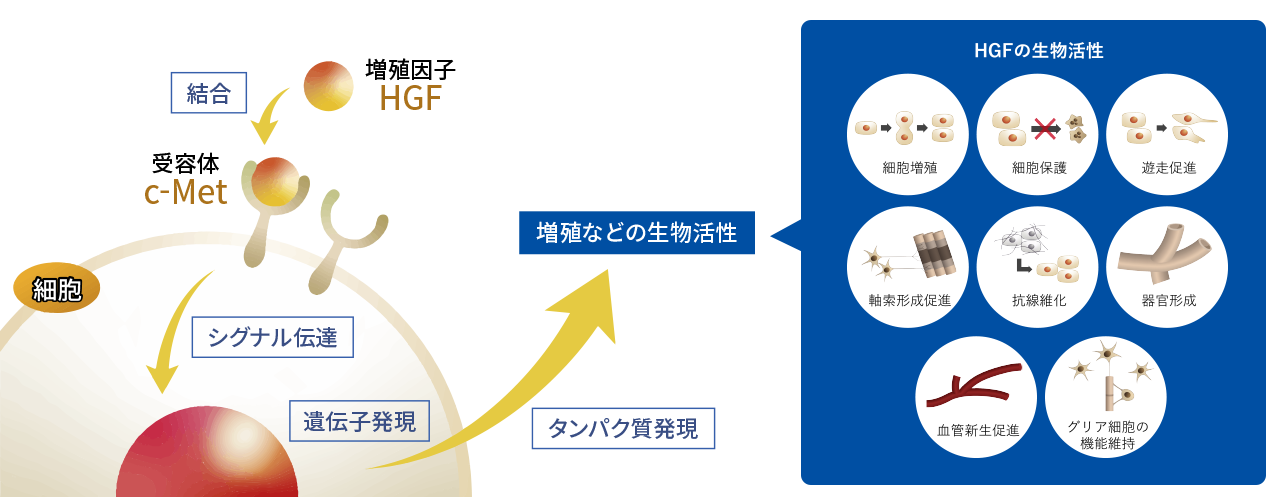
Subsequent studies have shown that HGF has multiple biological activities other than cell proliferation, and that it is effective not only for hepatocytes but also for cells in the kidneys, lungs, skin, and other organs. In particular, since its action of releasing fibrosis or sclerosis, in which cell functions are impaired due to the accumulation of fibrous components, and its biological activities on nervous cells such as neurons and glial cells were demonstrated, various research results have been reported for HGF as a potential therapeutic agent for several intractable diseases.
Development pipelines
- Clinical trials of HGF protein underway for multiple target indications
- Late-stage pipeline (Phase III: two studies, Phase II: one study)
- Resources devoted to development in acute phase spinal cord injury, vocal fold scar and ALS
We have four pipelines that have progressed to clinical trials, acute phase spinal cord injury, vocal fold scar, amyotrophic lateral sclerosis (ALS), and acute kidney injury, while our partner, Claris Biotherapeutics, is conducting one clinical trial for an ophthalmic disease. Several other programs are in the basic research stage. Currently, our focus is on the acute phase spinal cord injury program, the most advanced pipeline, and we are concentrating our resources to obtain regulatory approval in Japan.
| Priority | Target indication | Development stage | Clinical trial | Application approval | Marketing | ||
|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | |||||
| 1 | Acute Spinal Cord Injury (Acute SCI) | Phase I/II study (placebo-controlled, double-blind, controlled trial) completed, POC acquired, orphan drug approved, and Phase III study completed. | Completed | Completed |  |
||
| 2 | Vocal fold scar | Phase I/II study (open-label dose-escalation study) completed (investigator-initiated study), Phase III study (placebo-controlled double-blind comparative study) ongoing. | Completed | Ongoing |  |
||
| 3 | ALS | Phase II study (placebo-controlled, double-blind, investigator-initiated) termination, no statistically significant differences in primary or secondary endpoints, additional analysis ongoing. | Completed | Completed |  |
||
| 4 | Acute kidney injury | Phase Ia, b Study (Open-Label Dose-Escalation Study) completed. Safety and pharmacokinetics confirmed. Partner search ongoing. | Completed | Searching for a partner |
|||
* LPO : Last Patient Out
** FPI : First Patient In
As of Feb 2025
Kringle Pharma’s growth strategy
We have two strategic pillars of business growth. The first is to have our own products. Our basic policy is to develop drugs for rare diseases and secure marketing approvals on our own, aiming to maximize value in the mid to long term. Spinal cord injury and vocal fold scar pipelines fall into the first pillar. The second is to expand globally. We develop innovative drugs for intractable diseases for which there are significant unmet medical needs. They should serve patients suffering from those intractable diseases not only in Japan but also around the world. We will be able to accelerate our growth by gaining overseas partners and reaching out to global markets as soon as possible.

* This is an overall plan, and there is no guarantee that the development will progress as shown in this graph.
Joint research with academia:
Pioneering new markets for novel regenerative treatments
In order to become a full-fledged biopharmaceutical company and achieve sustainable growth, while we promote our pipeline development, we actively engage in joint research with academia (universities, etc.) to pursue further potential of HGF proteins. Japanese academia has a broad range of excellent seeds and technologies related to regenerative medicine. By combining these with HGF protein, we aim to identify innovative drug candidates for regenerative medicine that will pave the way for the future new market.
Professor Hideyuki Okano, M.D., Ph.D. Keio University Regenerative Medicine Research Center
Professor Masaya Nakamura, M.D., Ph.D. Department of Orthopedic Surgery, Keio University School of Medicine
- Development of optimal next-generation therapies for the acute, subacute, and chronic stages of spinal cord injury
- Combination therapy of iPS cell-derived neural stem/progenitor cells and HGF protein
Professor Shigeru Hirano, M.D., Ph.D. Department of Otolaryngology-Head and Neck Surgery, Graduate School of Medical Science, Kyoto Prefectural University of Medicine
- Clinical development for vocal fold scar
- Research on the mode of action for the treatment of vocal fold scar and the next-generation treatment strategy
Professor Masashi Aoki, M.D., Ph.D. Department of Neurology, Course of Neuro-sensory Pathology, Graduate School of Medicine, Tohoku University
- Clinical development in ALS
Professor Ryuichi Okamoto, M.D., Ph.D. Department of Gastroenterology and Hepatology, Institute of Science Tokyo
- Clinical research on regenerative medicine using autologous intestinal epithelial stem cell transplantation
- Use of HGF protein in the preparation of intestinal epithelial organoids for transplantation therapy
Professor Yasuhiko Tabata, M.D., Ph.D. Institute for Life and Medical Sciences, Kyoto University
- Search for optimal and effective next-generation regenerative therapies for target diseases
- Combination of biomaterials and HGF protein
Professor Kunio Matsumoto, Ph.D. WPI-Nano Life Science Institute, Kanazawa University
- Research on next-generation manufacturing methods for HGF protein
Professor Haruhiko Akiyama, M.D., Ph.D. Department of Orthopaedics, Gifu University School of Medicine
Associate Professor Ryuta Asada, Ph.D. Advanced Medical and Clinical Research Promotion Center, Gifu University School of Medicine
- Research on the Application of HGF to Idiopathic Femoral Head Necrosis
Professor Seiji Yano, M.D., Ph.D. Graduate School of Medical Sciences, Kanazawa University Respiratory Medicine
Associate Professor Satoshi Watanabe, M.D., Ph.D.
- Research on the Application of HGF to Idiopathic Pulmonary Fibrosis
Assistant Professor Narihito Nagoshi, M.D., Ph.D. Department of Orthopedic Surgery, Keio University School of Medicine
- Exploration of Biomarkers for brand-new acute stage predict spontaneous recovery after spinal cord injury.